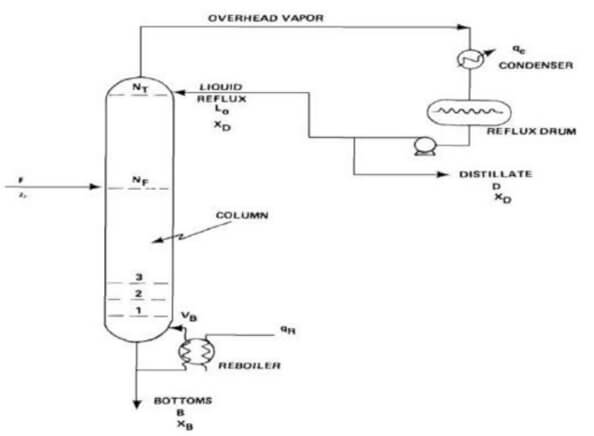
Distillation is a process that separates two or more components into an overhead distillate and bottoms.
The bottoms product is almost exclusively liquid, while the distillate may be liquid or a vapour or both.
The separation process requires three things. First, a second phase must be formed so that both liquid and vapour phases are present and can contact each other on each stage within a separation column.
Secondly, the components have different volatilities so that they will partition between the two phases to different extent.
Lastly, the two phases can be separated by gravity or other mechanical means.
Distillation differs from absorption and stripping in that the second phase is created by thermal means.